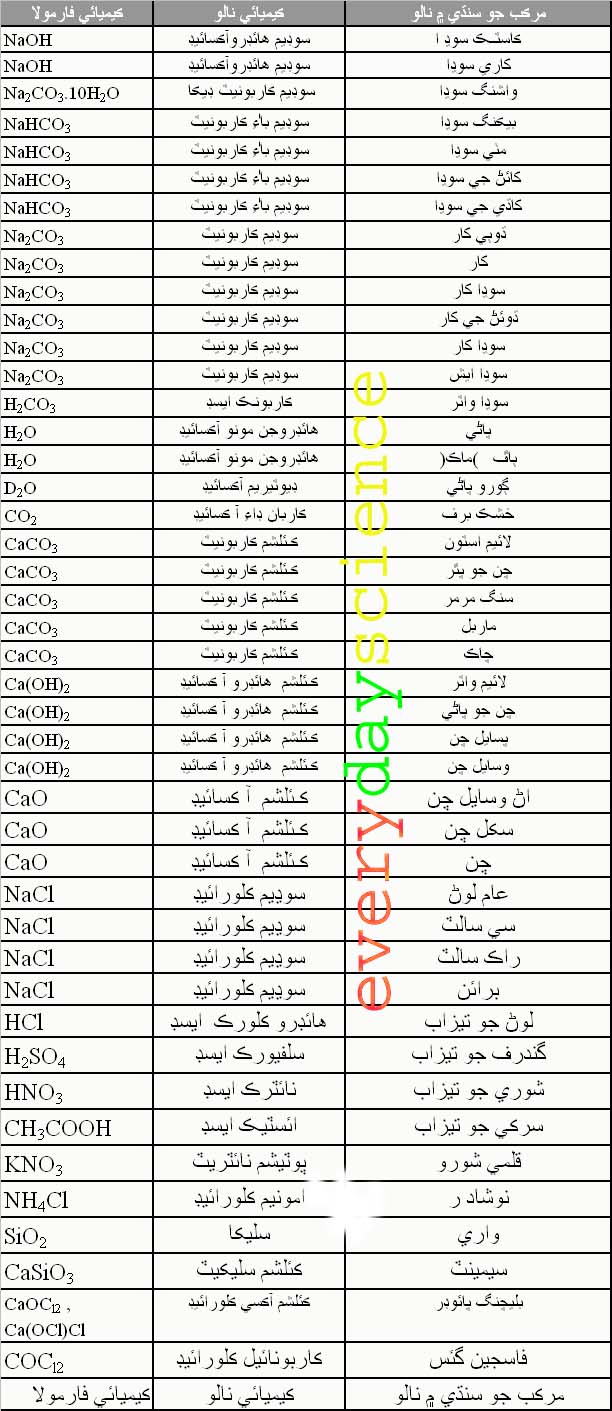
Everyday Science CHEMICAL FORMULAS
Timestamps:0:00 Introduction0:58 Formula Density1:14 Formula Temprature Unit Conversions3:00 Formula Mass of one atom3:22 Formula Average atomic mass4:14 For.

Chemistry formula for class 11 chapter some basic concept of chemistry
Unit 1 Some BaSic conceptS of chemiStry Science can be viewed as a continuing human effort to systematise knowledge for describing and understanding nature. You have learnt in your previous classes that we come across diverse substances present in nature and changes in them in daily life.

Chemistry Formulas Cheat Sheet Teaching chemistry, Chemistry notes
In this lecture, I will teach you the full chapter of some basic concepts of chemistry class 11. You will learn all the important topics of basic concepts of.

Chemistry Class 12, Chemistry Basics, Chemistry Study Guide, Chemistry
'Some basic concepts of chemistry' is the most fundamental chapter of complete chemistry. It gives information about the atomic number and mass number of elements.

Some Basic Concepts Of Chemistry Formula Sheet
Download PDF Download CBSE Class 11 Chemistry Formulae in PDF format. All Revision notes for Class 11 Chemistry have been designed as per the latest syllabus and updated chapters given in your textbook for Chemistry in Class 11. Our teachers have designed these concept notes for the benefit of Class 11 students.

Some Basic Concepts of Chemistry Class 11
Class 11 Chapter 1 Some Basic Concepts of Chemistry is an introductory chapter but very crucial to understand for students as it forms the basis of Chemical reactions happens around us. To understand these concepts, Class 11 Chapter 1 Chemistry Notes are prepared by subject experts in well-defined and easy language.

Pin on dvpmt perso
The metal, having lost one or more electrons, forms a cation, an ion with a positive charge; the nonmetal, having gained one or more electrons, becomes an anion, an ion with a negative charge. When two elements form a covalent bond, one or more electron pairs are shared between these two elements.
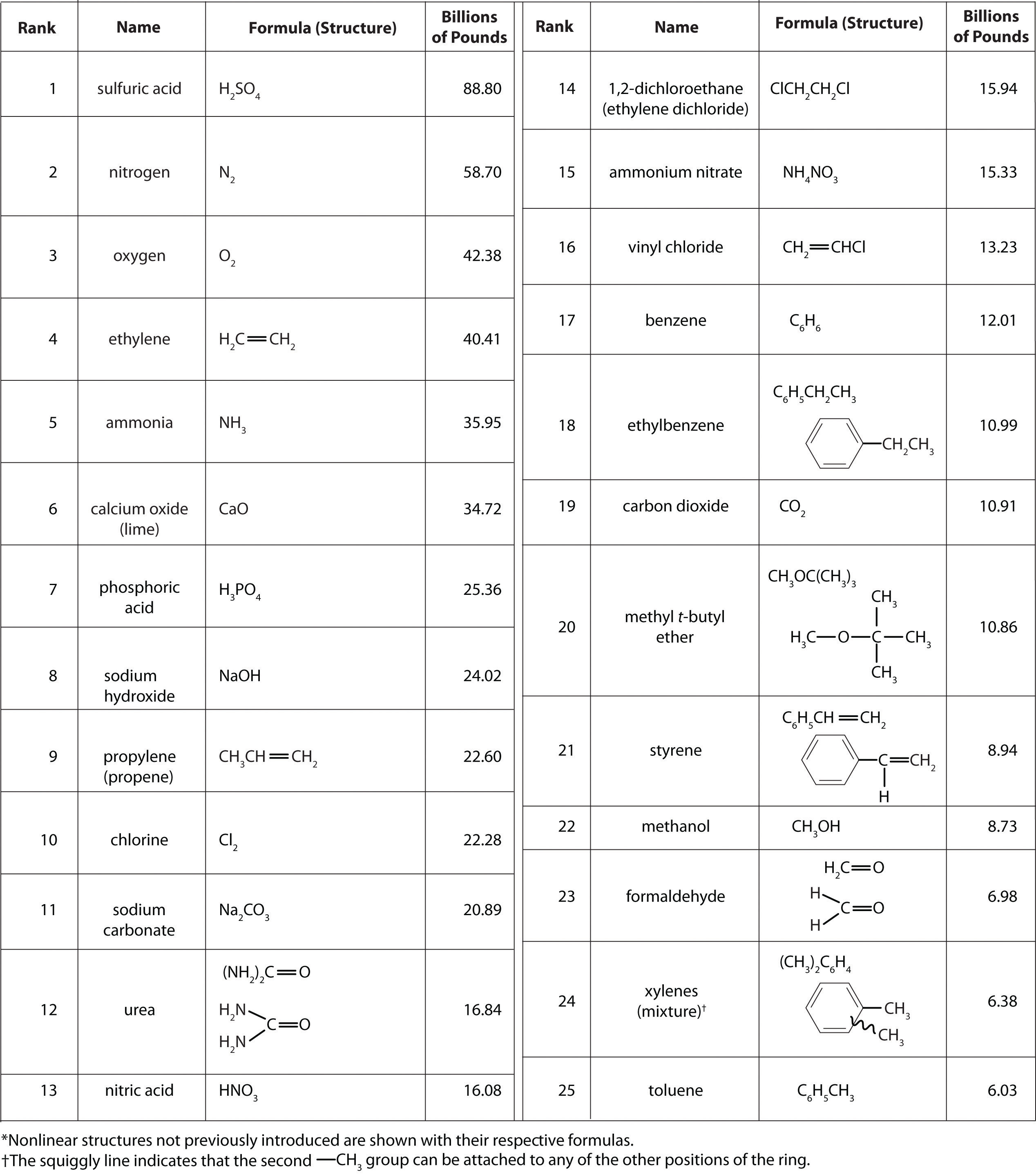
Molecules, Ions, and Chemical Formulas
"Some Basic Concepts of Chemistry" is the first chapter in the Class 11 Chemistry syllabus as prescribed by NCERT. The chapter touches upon topics such as the importance of Chemistry, atomic mass, and molecular mass.

Some basic concept of chemistry Class 11 formula PW
Molecules represent the basic unit of a chemical compound, and this another of those essential basic chemistry concepts. That's basically it. An example is a water molecule, which is made from two atoms of hydrogen (H) and one atom of oxygen (O), held together via covalent bonds. Models of a water molecule.

Chemistry Formulas Chart HSC Higher Secondary Education Website
Answer: (i) Molecular mass of H 2 O = 2 (1.008 amu) + 16.00 amu=18.016 amu (ii) Molecular mass of CO 2= 12.01 amu + 2 x 16.00 amu = 44.01 amu (iii) Molecular mass of CH4= 12.01 amu + 4 (1.008 amu) = 16.042 amu Question 2. Calculate the mass percent of different elements present in sodium sulphate (Na2 SO4). Answer: More CBSE Class 11 Study Material

Pin by Joseph Quattrocchi on Phillips Academy Chemistry review
Some basic concepts of chemistry | Khan Academy Class 11 Chemistry (India) 13 units · 107 skills Unit 1 Some basic concepts of chemistry Unit 2 Structure of atom Unit 3 Classification of elements & periodicity in properties Unit 4 Chemical bonding and molecular structure Unit 5 States of matter Unit 6 Thermodynamics Unit 7 Equilibrium
Some Basic Concepts of Chemistry Notes ScienceMotive
Introduction to Some Basic Concepts of Chemistry We'll look at the structure of atoms as well as the subatomic particles that make up atoms in this chapter. To understand the bond formation, one must first appreciate the electronic structure of atoms or the arrangement of electrons around the central nucleus.
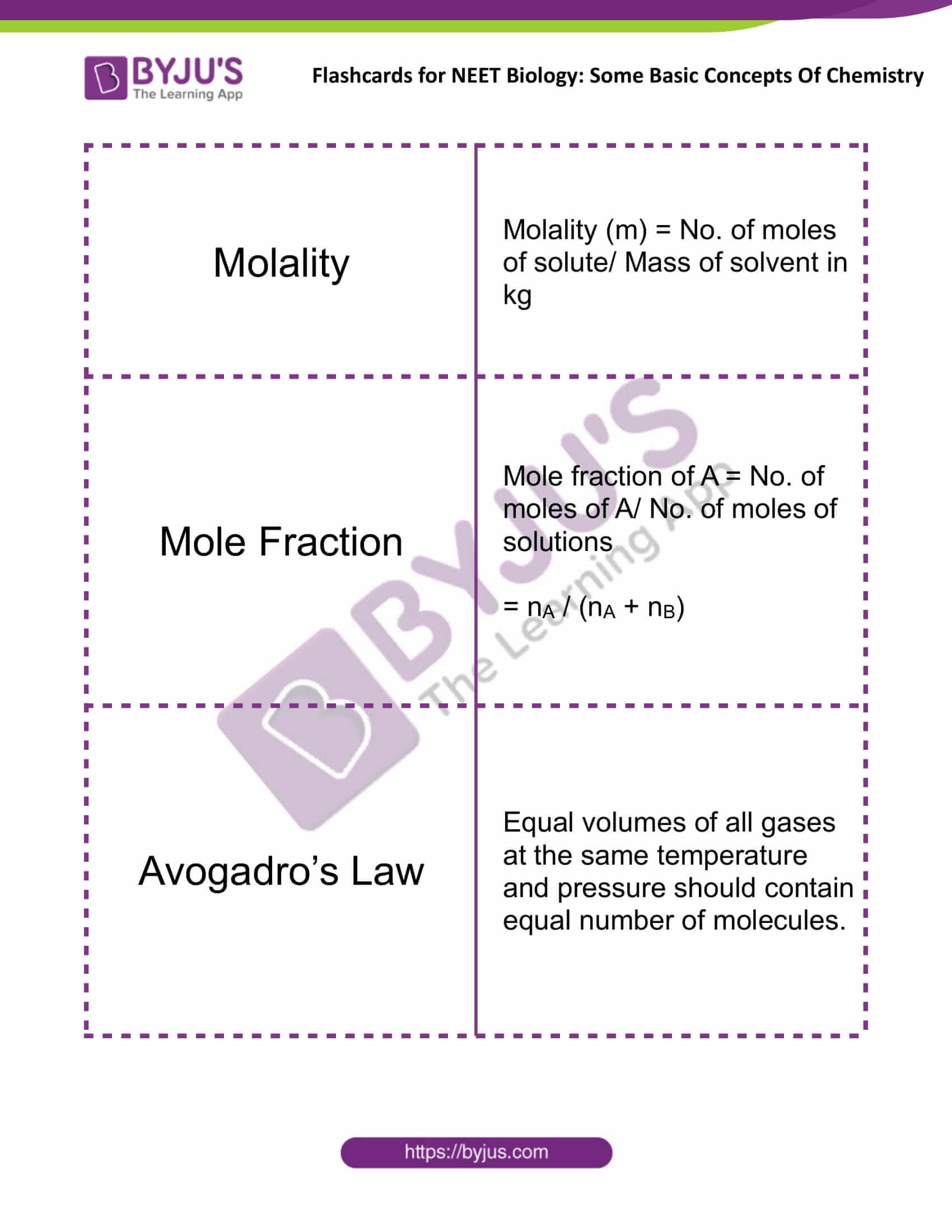
Some Basic Concepts of Chemistry Flashcards for NEET Chemistry
That's great! But even if you are not, you must know some basic concepts of Chemistry. I'm sure after learning these, you might become a fan of Chemistry. Let's learn about what Chemistry actually is and it's basic concepts that will help you understand Chemistry a lot better. Atomic Mass and Molecular Mass. Concentrations. Dalton's.

Some basic concept of chemistry Class 11 formula PW
Solution. The H:C ratios for the two alcohols are 4:1 = 4.0 for methanol and 6:2 (3.0) for ethanol. Alternatively, one sometimes uses mole fractions to express the same thing. The mole fraction of an element M in a compound is just the number of atoms of M divided by the total number of atoms in the formula unit.
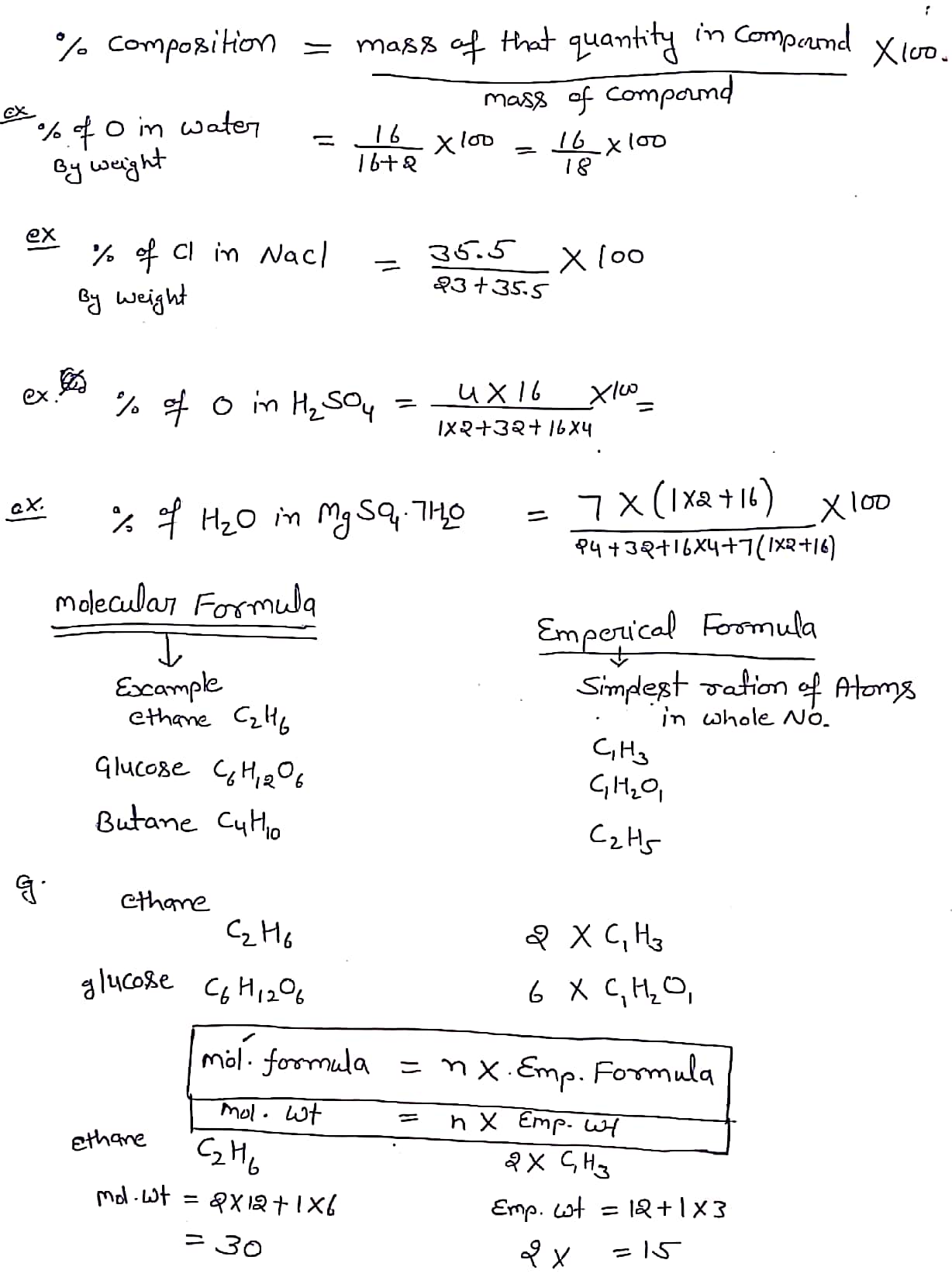
Some basic concepts of chemistry notes Studypur
Chemistry formula for class 11 chapter- some basic concept of chemistry Jul 05, 2022, 16:45 IST Matter is substance which has mass and occupies space, e.g., water, air, box, table, etc. State of Matter: Solid, liquid and gaseous state represent three different states of mater.

Introduction to some basic concepts of chemistry YouTube
CBSE Class 11 Notes Chapter 1 - Some Basic Concepts of Chemistry Laws of Chemical Combination Frequently Asked Questions on CBSE Class 11 Maths Notes Chapter 1 Some Basic Concepts of Chemistry Chemistry is referred to as the "Central Science" as it interconnects geology, biology, environmental science, and physics to each other.